
Behind the Development of “EXO.ECT.”
※This video is a shortened version. Please contact us if you would like to see the full version.
Living organisms have evolved through the adaptation of cells in response to stimuli from the natural world, known as the hormesis effect. This includes evolution from the global ice age to the Cambrian explosion of life. In other words, it can be said that by being subjected to intense stress, organisms are able to adapt to or transcend that stress, furthering their evolution. This power of adaptation has been applied to stem cells and developed into ‘EXO.ECT.’
We have devised a method—Patent Number: JP6985703—to stimulate mesenchymal stem cells in their natural cold-temperature environment without any cell manipulation using drugs or genetic engineering. This method safely isolates cell growth factors using a filter.
Technical Overview
Live Extracted Stem Cell Culture is a technology designed for “EXO.ECT.” (scientific name) in humans. It is derived from stem cells but has important differences.
Cells have a cell membrane that controls the movement of substances in and out of the cell. “Live Extracted Stem Cell Culture” is a collective term for substances extracted from the interior of stem cells after breaking down the cell membrane using a special technique and then filtering to remove cell components such as the nucleus.
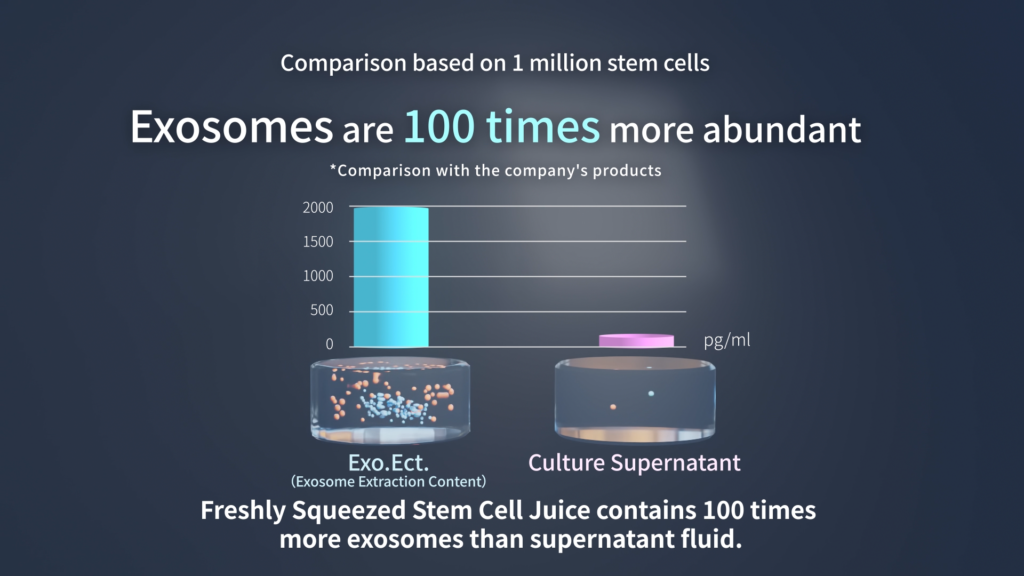
The Difference Between “Stem Cell Culture Supernatant” and “EXO.ECT.”
Specialized liquid is used for the cultivation of stem cells, which is referred to as a “cultivation medium.” The composition of this medium varies among different companies, and the specific components it contains are known only to the developer of that specific medium. This is because the cultivation medium is an integral part of the growth technique itself, representing the culmination of significant time and development expenses. Therefore, it is typically not openly disclosed.
The cultivation medium typically contains various artificially created growth factors and additive factors. This is because the type and quantity of these factors can influence how stem cells are cultivated. Many growth factors are commonly produced using low-cost organisms such as E. coli. As a result, the cultivation medium not only contains specific growth factors but also a mixture of various other factors. Additionally, the cultivation medium often includes additives, some of which may not be broken down by the body.
Furthermore, cultivation media for stem cells can include components such as serum from animals like cows and pigs. This addition of animal-derived serum makes it easier for cells to proliferate. The use of animal-derived serum, however, is prohibited for regenerative medicine in human healthcare due to the potential risk of contamination by unknown viruses and other factors.
However, in stem cell culture supernatant—which is available as a cosmetic ingredient—there are no specific regulations regarding the presence of animal-derived serum. To cultivate stem cells, an animal-derived cultivation medium is used, and during the proliferation process the stem cells secrete other factors. These factors include growth factors and extracellular particles like exosomes.
In essence, the stem cell culture supernatant contains factors derived from stem cells within the cultivation medium. This medium contains a substantial amount of artificial additives. In other words, it can be thought of as a combination of artificial additives and factors derived from stem cells. Among these factors, there are trace amounts of exosomes which have gained attention in recent years. They are present in minimal quantities because they are released outside the cells due to stimulation during the cultivation process.
In stem cells and in all other cells, there is a cell membrane that controls the exchange of substances between the interior and the external environment. The exosomes contained in the supernatant are those that have been released outside the cells through the cell membrane.
What is EXO.ECT. or Live Extracted Stem Cell Culture?
In the manufacturing process of EXO.ECT, stem cells are grown using a cultivation medium that does not contain serum. The cultivation of stem cells subjects them to a considerable amount of stress. During this process some cells may die and only the surviving cells are collected. These surviving cells undergo a repeated cycle of freezing and thawing to break down the cell membrane.
In this process, stress is applied to the cells solely through temperature differences without the use of artificial additives or chemicals. This stress helps extract substances within the stem cells, such as proteins and exosomes.
Safety Considerations
This technology has been validated for its use in the realm of reproductive medicine, where the highest level of safety is demanded. It has been shown to enhance sperm activity, improve the accuracy of artificial insemination, and have no adverse effects on factors like the number of offspring or the occurrence of deformities.
In other words, it exclusively contains substances derived from stem cells, making it essentially a 100% pure stem cell-derived substance.
However, EXO.ECT. is not made up of cells because it does not contain components like mitochondria or DNA. Therefore, it is considered safe even though it is derived from other cells.
| Stem Cell Culture Supernatant | EXO.ECT. | |
| Artificial Additives | Included | NO |
| Artificial Growth Factor | Possibly included | NO |
| Serum | Possibly included | NO |
| Factors Derived from Stem Cells | Small quantities | 100% |
Contact
After you submit your inquiry, an automated confirmation email will be sent to you by the support office.
If you do not receive the inquiry confirmation email within 24 hours, please take the trouble to check your spam folder.
Please note that it usually takes about 3 business days for us to respond to your inquiry.
<About Us>
- Company Name:
-
Advanced Medical Gateway Co., Ltd.
- Address:
-
7th Floor, 1-5-7 Nihonbashi Horitomecho, Chuo-ku, Tokyo 103-0012, Japan
- Phone Number:
-
TEL +81 03-6661-7987

